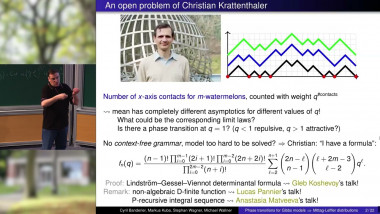
Appears in collection : Stochastic Processes in Evolutionary Biology / Processus Stochastiques en Biologie Evolutive
Genetic differences are a critical driver of disease risk and healthy variation, across the tree of life. Mutations arise and spread in our distant, genealogical ancestors, and so genetic variation data can provide a window into our evolutionary past, allowing us to understand processes such as population size changes, admixture, natural selection, and even evolution of the mutation and recombination processes that generate the variation itself. It has long been recognised that knowledge of genealogical relationships among individuals would allow us to capture almost all the information available from such data. However, only in recent years has it become computationally feasible to infer such genealogies, genome-wide, from variation patterns. One such method, Relate, developed in our lab, allows approximate inference of genealogical trees under coalescent-like models, for up to tens of thousands of samples. Here, we will show that a powerful approach for inference is to identify and characterise departures from the relatively simple models used to build these trees. By defining a 'population' as a set of coalescence rates between labelled individuals backwards in time, we can uncover variability in these rates, and use a single collection of trees to identify ancient mixing events among populations - including 'ghost' groups we have never sampled - natural selection favouring the descendents of particular branches of the genealogy, and departures from mathematical expectations under clock-like behaviour, indicating disruption of recombination or mutation.